Understanding how molecules like HCOOCH₃, CH₂, and H₂O interact opens the door to some truly exciting parts of organic chemistry. This combination of compounds doesn’t just represent a simple chemical equation—it’s a window into how everyday industrial chemicals are made, how reactions work on a molecular level, and why certain processes are more sustainable than others.
Let’s break this down in plain language and explore how HCOOCH₃ (methyl formate), CH₂ (methylene group), and H₂O (water) come together to create transformations that chemists rely on daily.
1. Getting to Know the Key Players
Before diving into the reaction, let’s understand what each component brings to the table.
-
HCOOCH₃ (Methyl Formate): This compound is an ester—a type of organic molecule formed when an acid reacts with an alcohol. It’s a colorless liquid with a pleasant smell and is often used in manufacturing formic acid, solvents, and perfumes.
-
CH₂ (Methylene Group): CH₂ isn’t stable when isolated—it usually appears as part of another molecule. Think of it as a “bridge” in larger organic compounds that can become reactive when conditions are right.
-
H₂O (Water): Besides being the universal solvent, water is a powerful participant in chemical reactions, especially in hydrolysis, where it helps break chemical bonds.
Together, these three form the backbone of important organic synthesis reactions, especially those involving ester hydrolysis and functional group transformations.
2. Breaking Down Their Behavior
Let’s imagine how these molecules behave in a reaction setting.
| Compound | Common Name | Functional Group | State | Main Role |
|---|---|---|---|---|
| HCOOCH₃ | Methyl Formate | Ester | Liquid | Reactant in hydrolysis and transesterification |
| CH₂ | Methylene Group | Reactive fragment | N/A (unstable free form) | Intermediate in organic synthesis |
| H₂O | Water | Polar molecule | Liquid | Hydrolyzing agent and solvent |
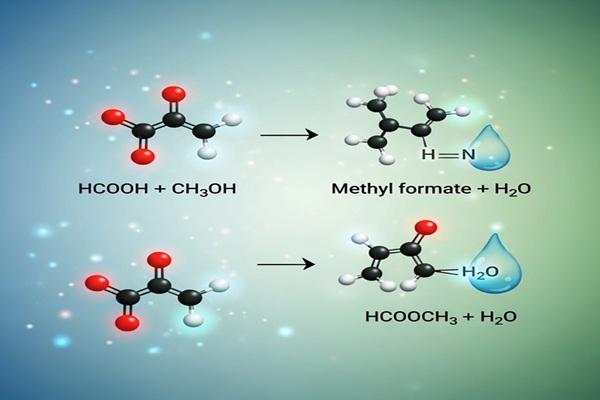
Each of these substances behaves differently based on reaction conditions like pH, temperature, and the presence of catalysts. For example, when methyl formate meets water in an acidic or basic medium, it tends to break apart through hydrolysis, forming formic acid and methanol—two compounds widely used in industry.
3. The Science Behind the Bonds
At the molecular level, methyl formate consists of a formyl group (HCO–) connected to a methoxy group (–OCH₃) through an ester linkage. This connection is somewhat fragile—it’s polar and vulnerable to attack by other molecules.
When water enters the picture, its bent shape and strong hydrogen bonds allow it to act as both a solvent and a reactant. It can break that ester bond through nucleophilic attack, resulting in the formation of formic acid and methanol.
Meanwhile, CH₂ often appears indirectly in these transformations—sometimes as part of a product like formaldehyde (CH₂O) that’s derived from oxidized methanol.
4. How the Reaction Works: Step-by-Step Hydrolysis
The HCOOCH₃ CH₂ H₂O reaction is often discussed in the context of ester hydrolysis, which can occur under acidic or basic conditions. Here’s how each pathway works:
Acid-Catalyzed Hydrolysis
Equation:
HCOOCH₃ + H₂O → HCOOH + CH₃OH
Conditions:
Dilute sulfuric acid (H₂SO₄), heat
In this setup, the acid donates protons to make the ester bond more reactive, allowing water to split it efficiently.
Base-Catalyzed Hydrolysis
Equation:
HCOOCH₃ + OH⁻ → HCOO⁻ + CH₃OH
Conditions:
Reflux with sodium hydroxide (NaOH) or potassium hydroxide (KOH)
Here, the hydroxide ion (OH⁻) from the base directly attacks the ester bond, releasing methanol and formate ions.
5. The Hidden Role of CH₂ in Organic Chemistry
Even though CH₂ doesn’t usually appear as a standalone reactant, it plays a vital role in organic synthesis. Think of it as a reactive “fragment” that helps connect molecules.
In some reactions, CH₂-like intermediates appear temporarily, influencing how new bonds form. For instance:
-
In the Reimer–Tiemann reaction, a similar reactive species adds to aromatic rings to produce formyl derivatives.
-
Methanol, formed from hydrolysis, can later be oxidized to formaldehyde (CH₂O)—a chemical cornerstone for making plastics, adhesives, and resins.
So, while CH₂ doesn’t stick around for long, it’s an essential link between simple starting materials and complex end products.
6. Real-World Uses and Industrial Importance
This combination of reactants has major industrial relevance. Here’s how each piece contributes to real-world chemistry:
-
Methyl Formate:
-
Used to manufacture formic acid, formamide, and pesticides.
-
Acts as a solvent for cellulose, resins, and oils.
-
Helps in environmentally friendly hydrolysis processes.
-
-
CH₂ Intermediates:
-
Play a part in producing polyethylene and formaldehyde resins.
-
Used to create synthetic materials and fuels.
-
-
Water:
-
Beyond being a solvent, it’s a green reactant that replaces toxic chemicals in sustainable reactions.
-
In short, the HCOOCH₃ CH₂ H₂O reaction isn’t just academic—it’s part of how industries create safer, more efficient, and eco-friendly chemical pathways.
7. Safety and Environmental Insights
Every chemical process comes with responsibilities.
-
Methyl formate is flammable and should be handled in ventilated areas.
-
CH₂ fragments are highly reactive and usually generated within a controlled system.
-
Water, while safe, can trigger unwanted side reactions if moisture-sensitive materials are present.
However, from an environmental perspective, using water as a reactant or solvent instead of harsh organic chemicals is a step toward greener chemistry. Many modern labs now prefer water-based reactions because they generate fewer hazardous by-products and require less energy.
As one industry chemist put it:
“Switching to water-based hydrolysis doesn’t just simplify reactions—it’s a commitment to sustainable science.”
8. Where Research Is Heading
Chemists are constantly looking for smarter ways to control these reactions. Some current trends include:
-
Using enzymes to catalyze ester hydrolysis under mild, energy-saving conditions.
-
Experimenting with ionic liquids and transition metal catalysts to increase yield.
-
Exploring photochemical techniques where light helps generate CH₂ intermediates for pharmaceutical synthesis.
These innovations show how a seemingly simple reaction like HCOOCH₃ CH₂ H₂O continues to inspire breakthroughs in green technology and sustainable chemical production.
Also Read : Acamento: A Comprehensive Guide to Meaning, Applications, and Future Possibilities
9. Hands-On Example: Simple Hydrolysis Experiment
Want to see ester hydrolysis in action? Here’s a safe laboratory-scale version often performed in organic chemistry labs:
-
Add 10 mL of methyl formate to a round-bottom flask.
-
Mix with 50 mL of distilled water and a few drops of dilute sulfuric acid.
-
Heat gently under reflux for about an hour.
-
Allow to cool and neutralize the solution with NaOH to confirm formic acid formation.
-
Distill the mixture to collect methanol.
This experiment demonstrates the principles of hydrolysis, reaction kinetics, and product recovery—the foundation of many industrial processes.
10. Clearing Up Common Misconceptions
Let’s set the record straight on a few things students often misunderstand:
-
“CH₂ is stable on its own.”
Nope—CH₂ only exists briefly as an intermediate. It’s far too reactive to exist independently. -
“Water is just a solvent.”
Water actively participates in hydrolysis and hydration reactions—it’s not just sitting in the background. -
“Ester hydrolysis is always slow.”
Not true. With the right catalyst, it can proceed surprisingly fast and cleanly.
11. Why This Reaction Matters
The HCOOCH₃ CH₂ H₂O reaction is more than just a line in a chemistry textbook—it’s an example of how simple molecules lead to valuable products.
-
Formic acid from this reaction is used in textiles, tanning, and as a reducing agent.
-
Methanol serves as a building block for fuels and plastics.
-
CH₂ derivatives contribute to materials science and polymer production.
Beyond the products themselves, this reaction embodies what chemists strive for: efficiency, safety, and sustainability.
12. Quick FAQs
Q1: What is HCOOCH₃ used for?
Methyl formate is commonly used to produce formic acid, formamide, and certain industrial solvents.
Q2: Can CH₂ exist independently?
Not under normal conditions—it’s usually an unstable, short-lived intermediate.
Q3: Why is water necessary here?
Water acts as a reactant in hydrolysis, helping break down the ester into an acid and an alcohol.
Q4: Is methyl formate dangerous?
It’s flammable and should be handled carefully in well-ventilated spaces.
Q5: How does CH₂O fit into this reaction?
Methanol from the reaction can be oxidized to formaldehyde (CH₂O), a major industrial compound.
Final Thoughts
The HCOOCH₃ CH₂ H₂O reaction beautifully illustrates how chemistry connects theory with real-world application. From the lab bench to large-scale manufacturing, this reaction showcases the versatility of esters, the reactivity of methylene groups, and the sustainability of water-based chemistry.
By understanding how these molecules interact, we not only master a crucial organic reaction but also gain insight into the greener, more efficient chemistry of the future.


